How to Build a Lemon-Powered Clock
Power a digital clock by making a battery out of lemons.
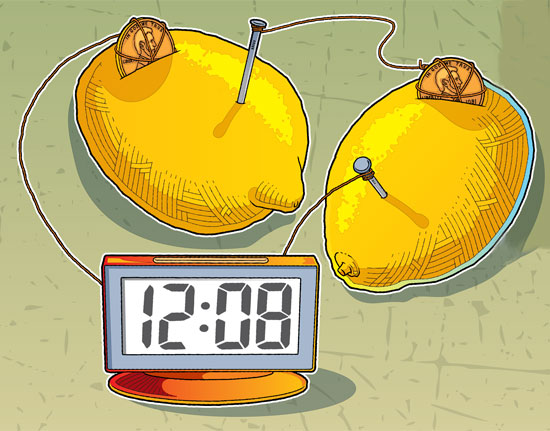
WHAT YOU’LL NEED
- 2 ripe lemons
- Low-voltage digital clock (Use a clock that takes one AA battery or a 1.5-volt button cell battery. One AA battery has about 1.5 volts of energy. Two lemons should produce about 1.5 volts.)
- 2 copper pennies (If your penny has a date before 1982, it is made of 95 percent copper. If the date is 1982 or later, it is made of 97.5 percent zinc with a thin copper coating. The old pennies work better.)
- 3 8″ lengths of copper wire
- 2 galvanized nails (Galvanized nails are coated with zinc.)
- Knife
- Scissors
- Clip leads (alligator clips), optional
WHAT YOU’LL DO
 1. Roll the lemons on a hard surface, being careful not to break the skin. (This will loosen the pulp, make the lemons juicy and help the electrons move through the lemons.)
1. Roll the lemons on a hard surface, being careful not to break the skin. (This will loosen the pulp, make the lemons juicy and help the electrons move through the lemons.)
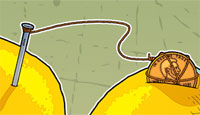 2. Wrap one end of a wire around a penny and the other around the end of a nail. (These will be used to connect the lemons together. Connecting lemons with metal wires adds voltage from each lemon. The more lemons you connect together, the higher the voltage.)
2. Wrap one end of a wire around a penny and the other around the end of a nail. (These will be used to connect the lemons together. Connecting lemons with metal wires adds voltage from each lemon. The more lemons you connect together, the higher the voltage.)
 3. Connect a second wire to a penny and leave the other end bare.
3. Connect a second wire to a penny and leave the other end bare.
 4. Wrap the third wire around a nail and leave the other end bare.
4. Wrap the third wire around a nail and leave the other end bare.
 5. Cut a slit in each lemon just large enough to insert the pennies. Insert the pennies and nails as shown in the image at the top of this page. (Make sure the copper wire has good contact with both the pennies and nails, and make sure the pennies and the nails make good contact with the lemon pulp and juice.)
5. Cut a slit in each lemon just large enough to insert the pennies. Insert the pennies and nails as shown in the image at the top of this page. (Make sure the copper wire has good contact with both the pennies and nails, and make sure the pennies and the nails make good contact with the lemon pulp and juice.)
 6. Remove the battery from the clock and touch the wires to the positive and negative terminals in the clock. If your clock doesn’t work, try switching the wires.
6. Remove the battery from the clock and touch the wires to the positive and negative terminals in the clock. If your clock doesn’t work, try switching the wires.
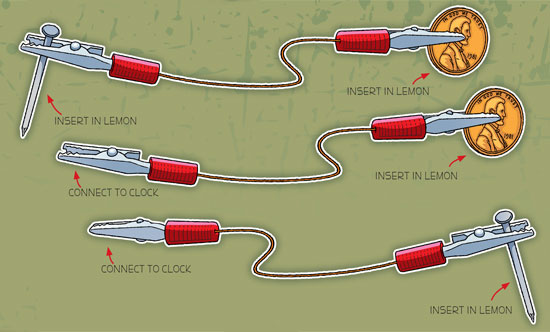
7. You can also use clip leads (alligator clips) to connect your wires to the pennies, nails and clock, as shown below.
PHOTOS OF COMPLETED PROJECT
Check out these photos of completed lemon-powered clocks sent to us by Boys’ Life readers. If you have a photos of a BL Workshop project, please use the form below to send them to us.

it seems cool, but do we need copper wires and copper coins?. Why not other metals?????
Copper is a good conductor so electricity will flow through easily
I know how to make this without looking it up but there is a different way you can make this
wahh is very creative, what content is there in the lemon so that it can produce electricity?
cool stuff DUDE!
Sweet I’m using this for my science final project!!!
would it still work if I used a clock that uses 2 AAA batteries but used 4 lemons???
So cool! I haven’t tried it yet but i think that the chemical reaction will work!!
great
not working
My daughter and I tried this with a lemon and a grapefruit, it kept our low voltage digital clock going for 21 days
Probably did the wire wrong or didnt have enough lemons attached or you probably made a mistake when making the project.
Why do we need the wire connecting the penny and nail? Can you please explain? Why can’t we just connect the positive and the negative of the battery to two different penny and nail and insetr them into the same lemon? The circuit will still be complete right??
Each piece of fruit is a single cell in your fruit battery. In order to connect the cells and produce more volts you need to use the additional second metal in each fruit and clip them together to complete the circuit.
mine didn’t work
Tried this experiment with two lemons, (no clips) everything else per the list and no success! Students will have to brainstorm and come up with reasons why it did not work!
still hasn’t worked
Can we use regular low voltage clocks because I cant find any low voltage digital clocks, i used other clocks that weren’t digital they didn’t work though
I did this for my science fair and I got first place so WHOO HOO!
Congratulations im doing it for my science fair
it will work
This is a pretty good project to do, sometimes though if the lemons don’t produce enough voltage the clock might not work. That’s the only downside to the experiment.
You can do that with potatoes too.
This did’t work for me. I first tried it with the lips. Don’t work. Then, the regular way. Didn’t work.
cool
It good
I love iiiiiittt
This is a bust i tried And tried And nothing
Test each lemon with a volt meter, I Had to use 3 lemons to get 1.5 volts but it worked. I ended up with 1.47 volts but the lemons were small. Experiment with zinc strip at different spacing, mine were 3/4″ apart. Fun experiment.
It made beaping noise but that means it worked
i will do that
thank you i have been so frusterated because mine werent working
it worked 🙂
Lol
Thanks helped alot with GCSE homework
That looks awesome but I can’t do it😥
Why!? It totes works, and got me a b+ in sci fair!
Haven’t tried but it sounds legible
That’s cool! I’m making one
It worked perfectly!
Did it work? I have a progect from school.
sad it didn’t work
Worked for me!
if it dident work give me some ideas. i got sci fair in a month
Well not to be rude, but if everyone says it does not work, I will not waste my time on it.
Does not work you just might get me a zero in the science fair
It didn’t work!!!
It doesn’t work I tried twice in class with the teachers help. Still doesn’t work.😭
that stinks
we did it but it didn’t work 🙁
that is sick and it works to!!
What side is Oxidation and what one is reduction?